How does the Omni work?
Discover how:The Omni is a live-cell analysis platform capable of continuous multiwell imaging directly from the incubator. Gain unprecedented access to dynamic biology and track cell health and function with automated, high-quality brightfield and fluorescent images of living cells. The versatile device can scan all types of flasks, microplates, or custom microfluidic chips while providing fast, reliable data analysis with powerful and intuitive software.
Key Features
-
>> Assay your cells in brightfield and fluorescence – From label-free cell monitoring to fluorescence-based assays, the Omni adds dynamic visual results to any experiment.
-
>> Track every moment, straight from your incubator – The Omni operates within an incubator, automatically capturing images as your cells grow in their optimal environment.
-
>> See every cell – The Omni moves the camera, not the cells, capturing detailed brightfield images of the entire culture without disturbing the cells.
-
>> Monitor and analyze your cells remotely – The software allows you to monitor your cells and perform data analysis from your desktop.
-
>> Get started quickly – With an easy-to-install, maintenance-free device that does not require calibration, a short training is all it takes to start using the Omni.
Overview
The Omni platform is designed to simplify and accelerate the acquisition of complex biological data. Using brightfield and fluorescence imaging combined with advanced software tools, the Omni can help you to examine cell health and function noninvasively and in real time.
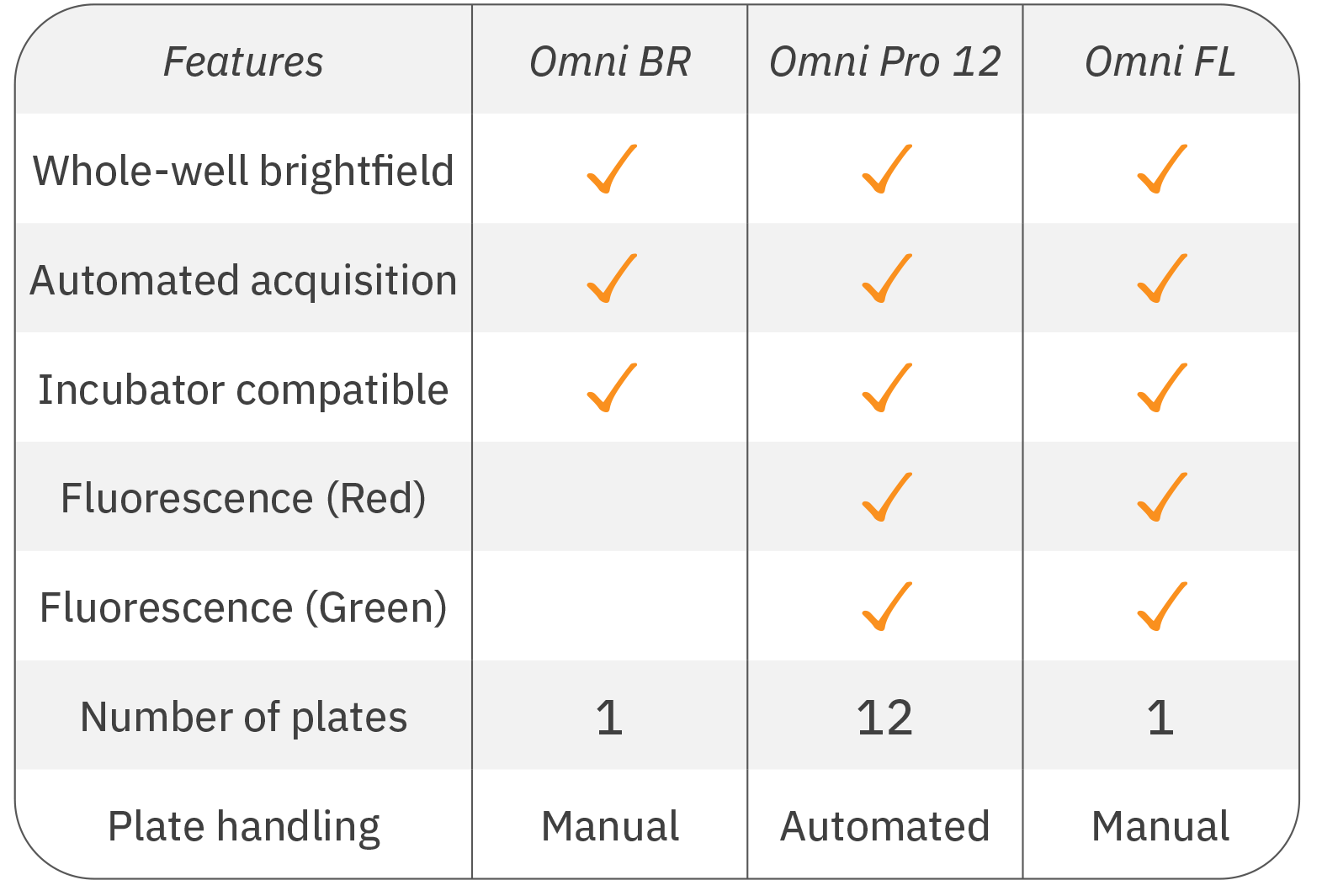
Software Modules
The Omni platform has five different software modules available to meet your assay needs:
Cell Confluence – Available in brightfield or fluorescence. Know exactly when to passage your cells or track cell growth and death in your experiments.
Clonogenic Assay - Monitor and quantify cell proliferation and colony formation to study the effects of cytotoxic and immunotherapeutic agents.
Fluorescent Object Count - Quantify fluorescent objects over time and between wells when running fluorescence-based assays.
FAQ:
Samples are illuminated using an LED and recorded with a moving camera positioned below the sample stage. During brightfield acquisition, the camera scans the sample stage and acquires a series of sequential images. One complete brightfield scan generates approximately 7850 snapshots. These are stitched to form an image with a surface area of 86 mm × 124 mm (3.4’’ × 4.9’’). When acquiring fluorescence images, users can choose how many snapshots of a defined location within the well to record. The images are uploaded to the Cloud where they can be analyzed using our image analysis algorithms or third-party software.
What type of image analysis modules can I use?
Details:The Omni is compatible with the following modules: Brightfield/fluorescence Cell Confluence Analysis Algorithm, Scratch Assay (i.e., collective cell migration) Analysis Algorithm, Clonogenic Assay Algorithm, and Fluorescent Object Count. Users always have the option to download the raw data and perform their own analysis on third-party software.
Can the Omni platform be used inside a cell culture incubator?
Yes,Yes, the Omni systems are designed to be used inside a cell culture incubator. The hardware and electronics can operate at 5-40°C and between 20-95% humidity.
What culture vessels are compatible with the Omni systems?
Vessel detailsAny transparent culture vessel that is lower than 55 mm (2.2’’) (height of the light arc) can be scanned. Some examples include 6- to 384-well plates, Petri dishes, and T25 to T225 flasks. Users should keep in mind that the size of the scan area is limited to 86 mm × 124 mm (3.4’’ × 4.9’’).

