Introduction
The use of hydrogels to mimic tissue is becoming increasingly common as they can mimic both the chemical and mechanical properties of tissues in vitro [1]. Furthermore, it enables the study of cells in 2D and 3D environments. For these reasons, hydrogels have been used to culture cells, spheroids and organoids [2]. However, using a hydrogel makes certain readouts challenging. This is especially the case for microscopy experiments where light has to pass through the hydrogel to a detector. Furthermore, cells do not always respond to hydrogels in a predictable fashion. Therefore, it is important to gain a better understanding of how cells interact with hydrogels through techniques like live-cell imaging. The CytoSMART® Lux3 FL is an automated live-cell imager that can be used for this purpose.

The aim of this study was to observe the shape, size and migration of cells cultured on a synthetic hydrogel. As a proof-of-principle human corneal keratocytes immortalized with simian virus 40 (HCK+ cells) were cultured on top of ureidopyrimidinone- hydrogels (UPy-hydrogels).
Materials and methods
UPy-hydrogels were made by combining monofunctional UPy molecules (UPy-glycinamide and UPy-cRGDfK; SymoChem) with bifunctional UPy molecules (UPy-PEG-UPy; SymoChem) at a ratio of 80:1 (2.66 wt%) or 160:1 (4.90 wt%) [3]. Monofunctional UPys were weighed and dissolved in 80 mM NaOH for 30 minutes at 70°C, 600 rpm after which the temperature was lowered to room temperature (RT), 600 rpm and the solution was neutralized with 1 M HCl. Bifunctional UPy-PEG-UPy was weighed and dissolved in phosphate buffered saline (PBS) for 120 minutes at 70 °C, 600 rpm after which the temperature was lowered to RT, 600 rpm. Mono- and bifunctional UPy solutions were mixed on ice in a volume ratio of 1:1, after which 10 µL of the mixture was pipetted into each well of a µ-Slide Angiogenesis (IBIDI). The resulting mixtures were allowed to gelate for 24 hours at 37 °C, 5% CO2 in a humid environment to prevent drying.
HCK+ cells (passage 20) were cultured on UPy-hydrogels in complete medium consisting of high glucose DMEM (Gibco), 10% FBS (Gibco) and 1% penicillin-streptomycin (Gibco)(37 °C and 5% CO2 ). The cells were transfected with 100 ppc CellLight™ BacMam 2.0 Actin-emGFP (Thermo Fischer Scientific). Samples were placed on a CytoSMART Lux3 FL and images were taken every 5 minutes for 72 hours. The migration of 56 cells was measured using the ImageJ plugin TrackMate [4] and analyzed with the IBIDI Chemotaxis and Migration tool. Shape and size of 52 randomly selected cells were measured by manually tracing cell outlines in ImageJ. Data was analyzed in GraphPad Prism v5.04. The differences between populations were analyzed using a Kruskal-Wallis test with a Dunn’s multiple comparisons test.
Results
It was demonstrated that the composition of the hydrogel played an important role in the presence of optical artefacts and the resulting transparency of the hydrogel. UPy-hydrogels with a ratio of 80:1 mono- to bifunctional UPys had large optical artefacts that made it impossible to bring cells into focus (Fig. 1A). UPy-hydrogels with a ratio of 160:1 mono- to bifunctional UPys had less optical artefacts (Fig. 1B and 1C). Therefore, it was possible to measure the shape, size and migration of HCK+ cells on these hydrogels (Fig. 2). Furthermore, it was demonstrated that fluorescent labeling of the cells was necessary to accurately distinguish the cells from the optical artifacts caused by the hydrogel (Fig. 1C).
It was discovered that HCK+ cells on the UPy-hydrogel were significantly smaller than those on polystyrene (p < 0.0001) (Fig. 2A). The aspect ratio of the two populations was not significantly different. However, the circularity was significantly different (0.01 ≤ p < 0.05) (Fig. 2B). There was only a slight difference in the median circularity, however the HCK+ cells on the UPy-hydrogel showed a greater variation in circularity. The average velocity of the HCK+ cells on a UPy-hydrogel was not significantly different from those of HCK+ cells on a polystyrene cell culture flask (Fig. 2C). This indicated that both the hydrogel and the staining likely did not affect HCK+ cell migration.
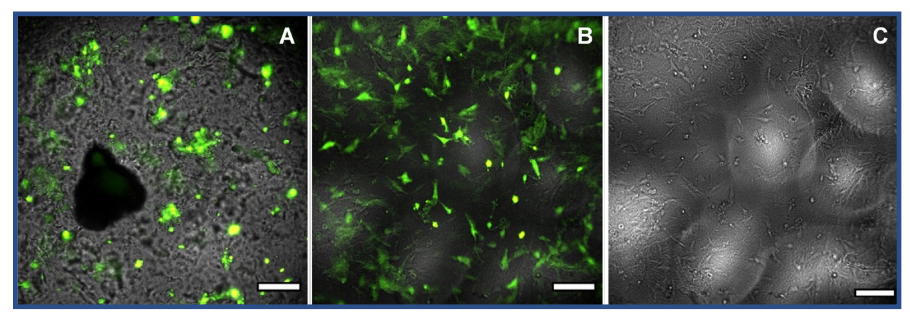
Fig. 1. HCK+ cells transfected with CellLight™ BacMam 2.0 actinemGFP on UPy-hydrogels with a mono- to bifunctional ratio of 80:1, wt% 2.66 (A) or 160:1, wt% (B and C) functionalized with 3.0 mM UPycRGDfK. The scale bar is 200 µm.
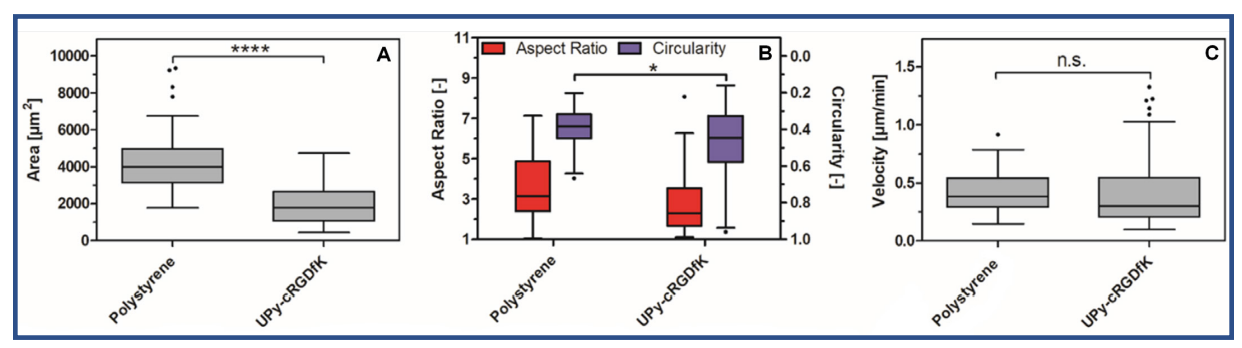
Fig. 2. The surface area A), Aspect ratio and circularity, B) and velocity, C) of HCK+ cells on polystyrene cell culture flasks and 160:1, 4.90 wt% UPyhydrogel. Populations that were deemed to be significantly different from one another using Kruskal-Wallis test with a Dunn’s multiple comparison test are denoted with an asterisk (*** p < 0.001, * 0.01 ≤ p < 0.05).
Discussion
In this study, we showed that it is possible to observe the behavior of fluorescently-labeled cells cultured on top of a hydrogel. It was demonstrated that the composition of the hydrogel played an important role in the optical properties of the hydrogel. Therefore, it is important to optimize the hydrogel to ensure high quality images can be made. However, the hydrogel also needs to be optimized for the used cells. Therefore, not every hydrogel composition may be suitable for live-cell imaging with the CytoSMART® Lux3 FL.
Next to this, we demonstrated that cells cultured on a hydrogel had a different shape and size than those cultured on polystyrene culture flasks. The implications of these differences were not investigated. However, it did demonstrate that the behavior of cells on hydrogels can be observed with the CytoSMART® Lux3 FL.
Conclusion
In summary, in this study we demonstrated that by combining a hydrogel with minimal optical artefacts and a fluorescent livecell staining the shape, size and migration of HCK+ cells on a hydrogel can be measured. Therefore, the CytoSMART® Lux3 FL could be a suitable device for real time monitoring of cell behavior in 2D on a hydrogel.
References
[1] Caliari, S. R., & Burdick, J. A. (2016). A practical guide to hydrogels for cell culture. Nature methods, 13(5), 405–414.
[2] Thakuri, P. S., Liu, C., Luker, G. D., & Tavana, H. (2018). Biomaterials-Based Approaches to Tumor Spheroid and Organoid Modeling. Advanced healthcare materials, 7(6).
[3] Bakker, M. H. & Dankers, P. Y. W. (2018) Supramolecular biomaterials based on ureidopyrimidinone and benzene-1,3,5-tricarboxamide moieties. Self-Assembling Biomaterials: Molecular Design, Characterization and Application in Biology and Medicine, 177–204.
[4] Tinevez, J. Y. et al. (2017) TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80–90.
