The clonogenic assay or colony formation assay is a well-established in vitro method for testing the survival and proliferative capability of cancer cells, and more recently cancer stem cells, under different treatment conditions1.
The assay assesses clonogenicity: the ability of a cell to clone itself and grow into a full colony of cloned cells2. This is typically an endpoint-based assay, where colonies are fixed and stained and survival curves are plotted (find out more about clonogenic assays here).
Although colony formation assay is more widely known as a cancer biology assay, it does have applications in the field of stem cell biology, where the self-renewal potential of stem cells and their progenitors is assessed3. Colony formation assays that are used in this context are not endpoint-based and rely on live-cell imaging. These assays enable the selection of desired colonies for further culturing and experimentation. Assessing colony formation is most commonly used for hematopoietic stem cells (HSCs), in the form of a colony formation unit (CFU) assay.
------------------------------------------------------
With CytoSMART Omni live-cell imager, you can analyze and quantify colony formation. Learn more about the Omni here.
------------------------------------------------------
This blog will focus on clonogenic assay applications in the field of HSCs.
Hematopoietic stem cells
Hematopoietic stem cell transplantation utilizing bone marrow as a stem cell source has become an accepted treatment mode for a variety of metabolic, immunologic, and hematologic disorders4. HSCs have the ability to differentiate into all hematopoietic lineages (Figure 1), while also maintaining their self-renewal capacity.
Although HSCs have the capacity to proliferate and differentiate in culture, most cells detected in hematopoietic culture assays consist of hematopoietic progenitor cells, which have limited self-renewal capacity and short-term hematopoietic potential.
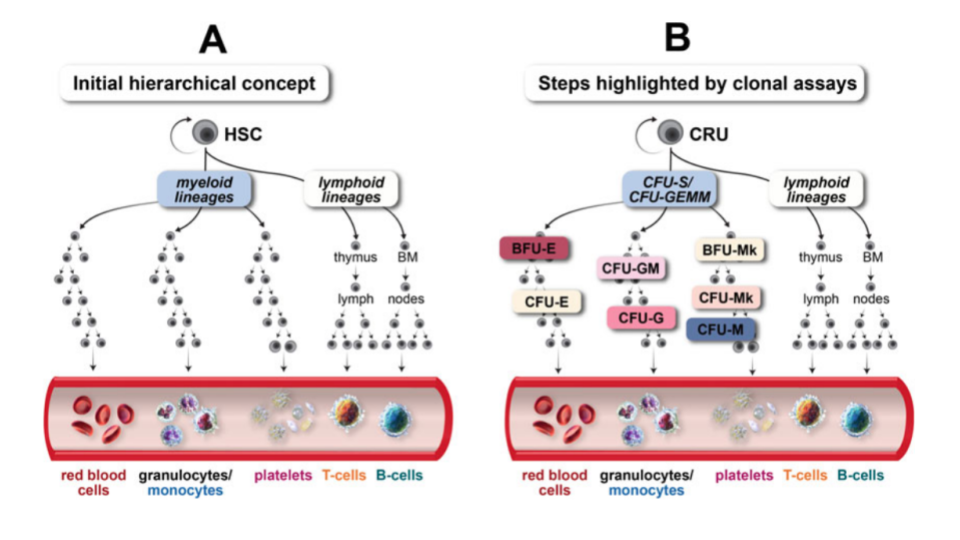
Colony formation unit assay
A clonogenic assay is the most direct quantitative method of measuring human hematopoietic progenitor cells in vitro. Hematopoietic colonies are essentially clones of cells produced by a single progenitor cell. The aim of colony-forming unit (CFU) assays is to define the potential of hematopoietic stem and progenitor cell populations for proliferation and lineage differentiation. Furthermore, the colonies can be morphologically analyzed (Figure 2)4.
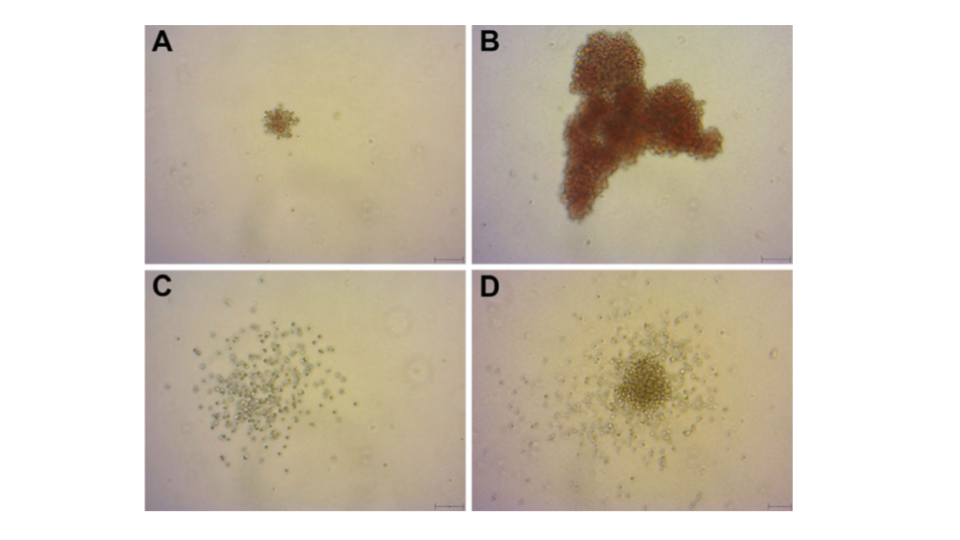
The selection of stem cell products for transplantation is typically based primarily upon non-functional cellular parameters, such as total nucleated cell counts and cellular immunophenotypes (e.g. CD34+ cell counts). However, it has been found that the absence of functional hematopoietic information can result in the selection of low potency stem cell products that fail to engraft in a patient despite a unit having a high cell count with an acceptable phenotype5.
The functional analysis of hematopoietic progenitor cells using a CFU assay enables measurement of the potency of self-renewal of hematopoietic progenitors present in stem cell products. This is critical for comparative selection of the highest quality stem cell product for a therapeutic application.
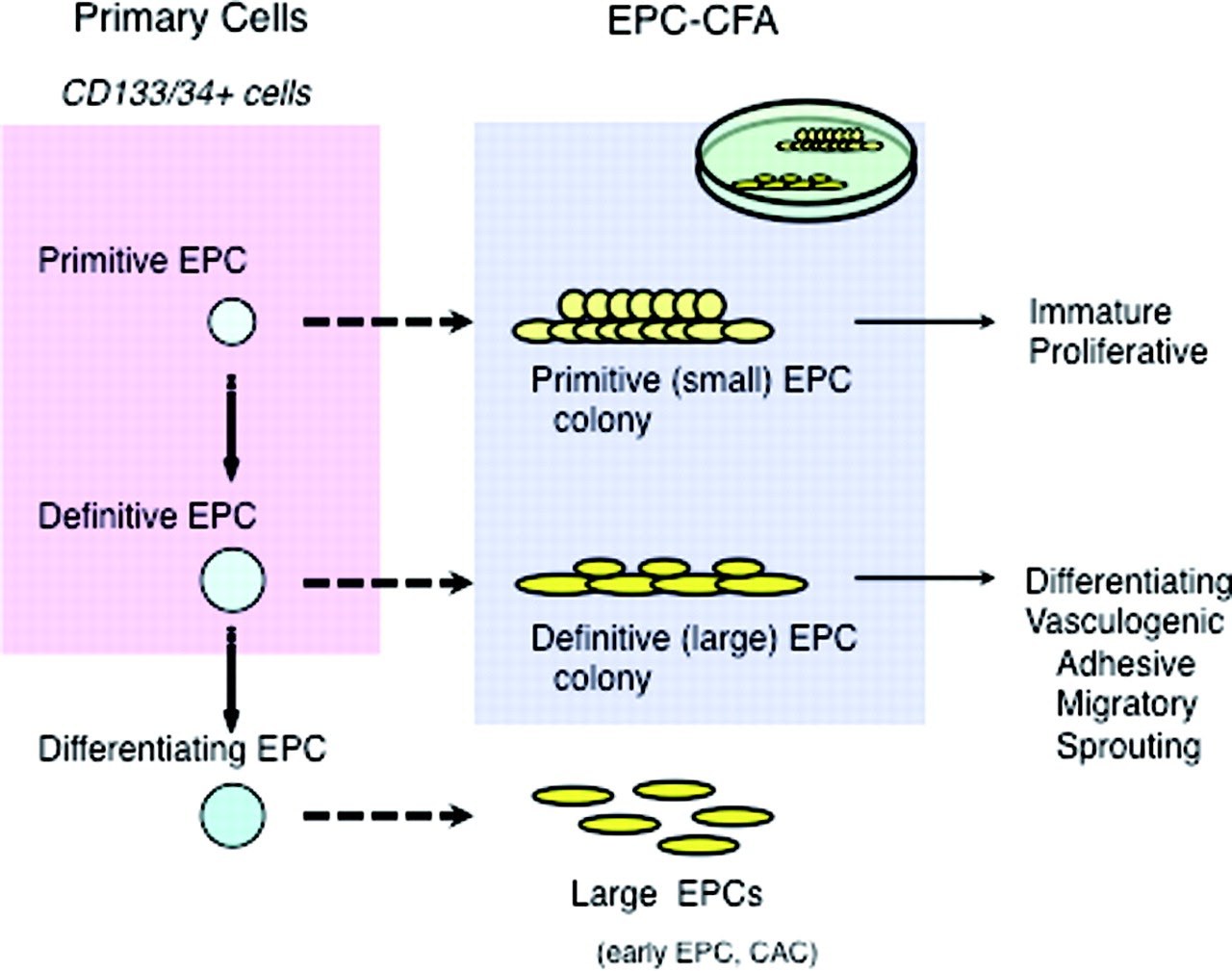
CD34+ or CD133+ HSCs give rise to two types of endothelial progenitor cell (EPC) colonies: primitive and definitive EPC-colony forming units, which can be morphologically defined. Based on their morphology, an evaluation of the number or the ratio of each EPC colony constitutes the endothelial progenitor cell clonogenic forming assay (EPC-CFA), an assay to quantify the differentiation of colony-forming EPCs. This assay system allows us to practically evaluate the vasculogenic potential of primary or cultured stem cell populations, i.e., mononuclear cells or fractionated stem cells (CD34+ or CD133+ cells) in peripheral blood, bone marrow, or umbilical cord blood.
EPC-CFA can be used not only for basic research in vascular biology but also for evaluating the vascular reparative activity of patients with cardiovascular diseases6. Masuda et al. (2011) describe a clonogenic assay system for the quantitative and qualitative analysis of human EPCs based on the EPC differentiation hierarchy (Figure 3). The authors found that small EPC colony-forming cells can be seen as “primitive EPC,” which are possibly derived from further immature and proliferative EPCs, and large EPC colony-forming cells can be seen as “definitive-EPC,” which are more prone to differentiate and promote EPC-mediated cell functions required for vasculogenesis. Definitive large EPCs are capable of differentiating into a non-colonizing large EPC phenotype7.
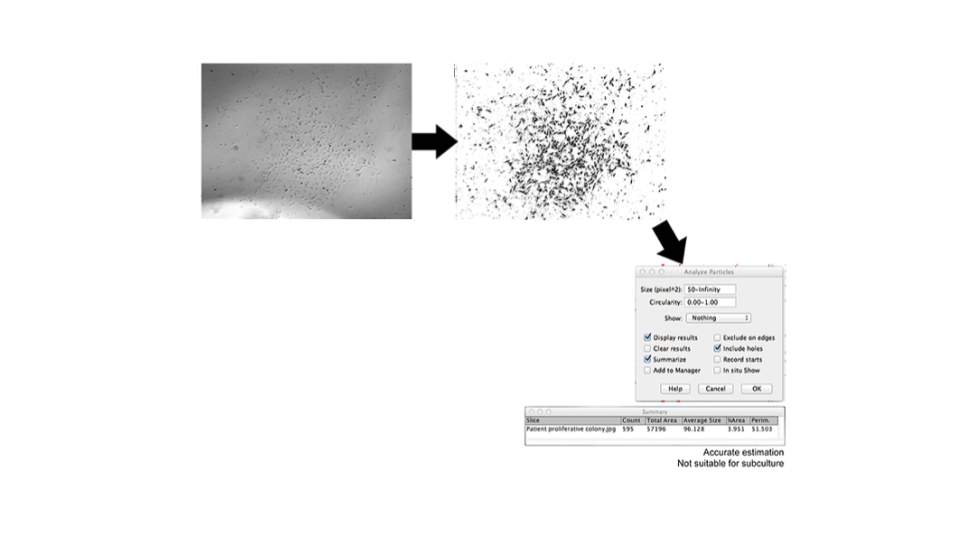
Endothelial colony-forming cells (ECFCs) are a subset of EPCs and are of interest as a possible therapeutic target for hypoxic diseases as they have high angiogenic potential. However, translation to clinical therapies has shown limited success8. This is partly explained by the difficulty of expanding EPCs into appropriate numbers due to senescence of the isolated cells. Huuskes et al. (2019), describe a method using live-cell imaging and automated image analysis to analyze the colony-forming ability of endothelial progenitor cells (EPCs).
Patient EPCs transform into ECFCs which are identified by morphology change, increased proliferative capacity, and the formation of an ECFC colony. Assessment of clonal expansion using live-cell imaging and automated image analysis to identify and inspect colony size enabled identification of ECFC colonies with angiogenic potential (Figure 4). This approach proved to be useful to correctly identify ECFCs ready for passaging to increase culture success and ultimately increase the therapeutic potential of ECFCs in treating vascular diseases9.
To conclude
Advancement in our understanding of the biology of adult stem cells and their therapeutic potential relies heavily on meaningful functional assays that can identify and measure stem cell activity in vitro and in vivo. Live-cell imaging technologies with automated colony formation analysis algorithms have the potential to be very beneficial for stem cell colony-forming/unit assays.
----------------
CytoSMART Technologies has developed an automated brightfield lab microscope, CytoSMART Omni that visualizes complete culture vessels and operates from inside a standard CO2-incubator. It has a built-in colony detection algorithm. Colony count, size, and circularity measurements are provided and can be used in the context of identifying types of colonies and their self-renewal potential.
------------------
Related Products
There are currently no products tagged to this resource.