Cell removal assay standard protocol
Cell removal assays are generally a low-tech solution to study cell migration and wound healing. The standard process entails damaging part of a confluent layer of cells, thus creating a cell-free zone in which cells can migrate. Thereafter, the cellular (and matrix) debris is removed by washing the sample with phosphate-buffered saline (PBS) or culture medium. As the last step, medium (supplemented with test compounds) is added to the samples, and the wound closure is measured. The wound size can be determined at the beginning and end of the assay, or at multiple time points throughout the assay. In the latter case, samples are either taken out of the incubator at each time point to take the images, or a live-cell imaging microscope is used with which the cells can be kept at constant temperature throughout the entire experiment.
The most cost-efficient and commonly used type of cell removal assay is the scratch assay, in which a pipet tip or other scratching tool is used to create a wound. However, many custom and commercially available variations of this assay have been developed in the last decade. These assays range from simply standardizing the mechanical scratch-making process to chemical wounding of the cell monolayer. Each of these methods has its technical requirements, reproducibility, and type of damage. The most common methods and their advantages, disadvantages, as well as commercially available products, are described below and summarized in Table 1 (below the text).
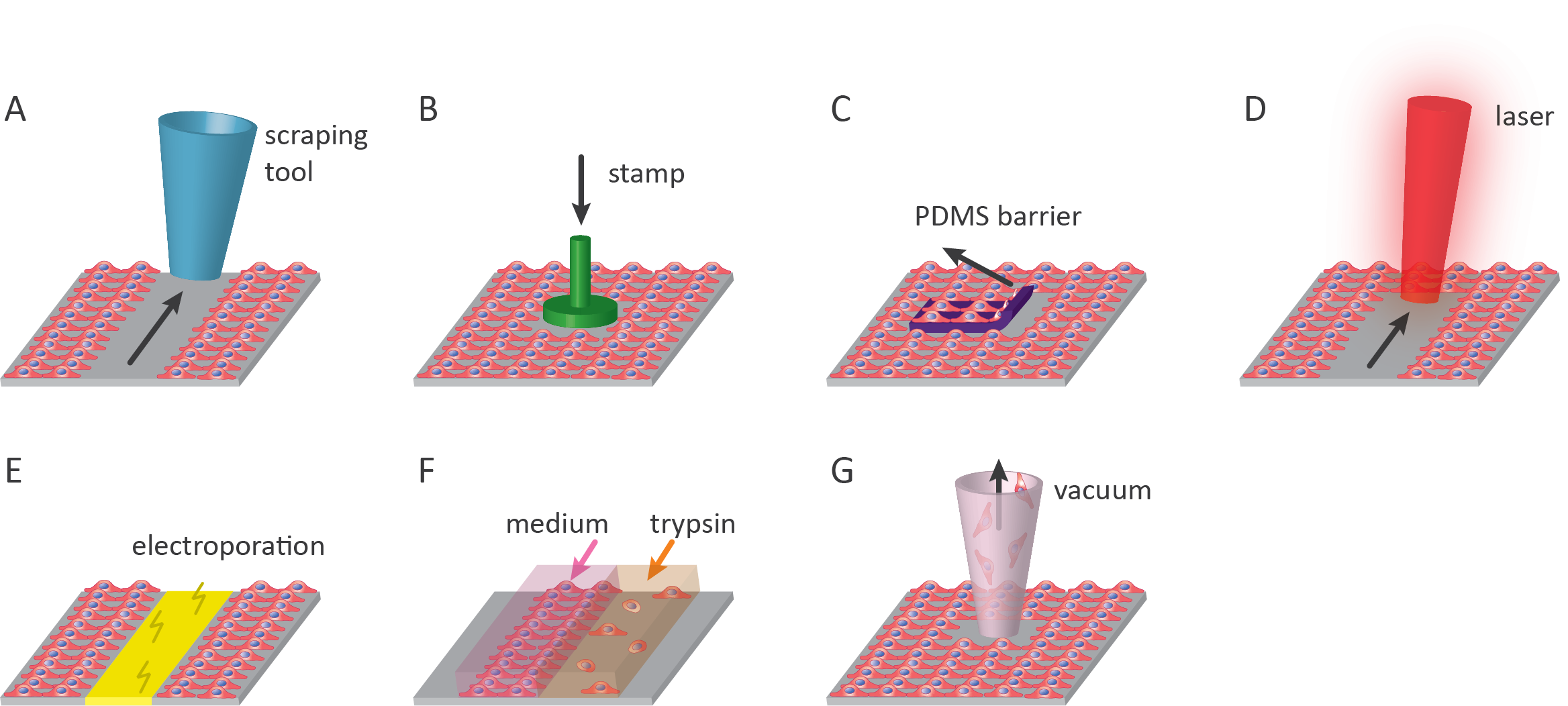
1. Scratch assay
The scratch assay is the most commonly used wound healing assay in which a confluent layer of cells is mechanically damaged since it is straightforward and affordable1,2. Next to this, the scratch assay can be performed using readily available lab tools ranging from pipet tips to metal spatulas1,3. The downside of removing cells in this manner is that it often disrupts the underlying extracellular matrix (ECM) coating on culturing surfaces. In addition to this, the accumulation of cells on the edge of the scratch can affect results when studying wound closure4. Mechanical scratches are mainly made by dragging a scratch tool from one side to the other, thus creating a linear scratch with a specific length and width. Therefore, this assay is limited in the range of void shapes that can be created. Although easy to implement, manual scratching often results in differences in the scratch shape and size between samples, which can severely affect the reproducibility between samples and experiments. To reduce variability, several commercial solutions have been developed. The most simple techniques comprise tools containing multiple identical pins that are moved simultaneously to create numerous comparable and reproducible scratches in one operation. Examples of these tools to form scratches of the same size are the Cell Comb™ Scratch Assay (Merck), IncuCyte® WoundMaker, HTSScratcher by Peira, and Wounding Pin Tools by V&P Scientific, Inc. More advanced tools, such as the BioTek AutoScratch™ make use of an automated system with multiple identical scratching pins to create comparable and reproducible voids. More examples of commercially available wound-making tools can be found in Table 1.
2. Stamp wounding
Stamping the confluent monolayer of cells is an alternative method to damaging cell cultures mechanically. Placing a weighted stamp on top of a layer of cells, either manually or automatically, destroys the underlying cells but often keeps the underlying substrate intact5,6. Another advantage of using stamps to wound the cell culture is that many different wound shapes and sizes can be created, in contrast to the linear wounds that can be created by using the scratch method. The type of stamp material mainly determines to what extent cells and debris will be removed from the cell culture area. Polydimethylsiloxane (PDMS) is commonly used for this reason, as cell debris easily attaches to it. However, caution should be taken as it is possible to disrupt the underlying structures severely, especially when applying too much pressure or when using specific stamp geometries that affect the pressure distribution on the cells7.
Stamp wounding can be integrated into microfluidic assays, as has recently been demonstrated by Sticker et al. (2017) and Kim et al. (2019). In both these microdevices wounding was generated using pneumatically controlled circular stamps8,9. Pneumatic control allows accurate control of the pressure of the stamp. Furthermore, the advantages of stamping in a microfluidic environment include higher reproducibility and robustness, the removal of cell debris during perfusion, and reduced assay duration. These microdevices also lend themselves to being automated further8,9.
Stamping can also be combined with heat. Wounding via localized heating can be performed using a thermo-mechanical combination of a heated stamping method10. This type of wounding is useful to study the wound healing after skin injuries in vitro. All thermal wounding inherently suffers from heat spreading away from the site of damage, making it challenging to create reproducible wounds10.
3. PDMS barrier
A variation of the scratch assay is the removal of a barrier from a monolayer of cells. In this assay, a hydrophilic PDMS slab is used as a barrier in the cell culture area11. When cells are seeded, they are allowed to grow and form a monolayer on the culture surface as well as on top of the PDMS barrier. Upon removal of the PDMS slab from the substrate, a void with wounded cells on the boundary is created for the cells to migrate into. This method of wounding is much more reproducible compared to creating scratches due to the standardized shape and size of the PDMS slabs of each sample. It is also possible to cut PDMS into any geometry of interest. This method is particularly useful to study cell-substrate interactions, because no matrix proteins are deposited by cells that are removed when creating the void11.
A primary disadvantage is that only hydrophobic PDMS will auto-adhere to dry and uncoated surfaces. However, to culture cells, the PDMS should be made hydrophilic, which increases the chance of cells and proteins protruding underneath the PDMS slab before removing it12,13. A minor disadvantage is that the PDMS slab needs to be attached and removed from the culture surface, which makes it difficult to automate this assay. No commercial products are available at the time of writing.
4. Laser-based wounding
Wounds can be generated using ablation by infrared (IR) or ultraviolet (UV) lasers. Depending on the method, custom void geometries can be produced. The creation of a cell-free zone via laser ablation offers high reproducibility and enables high throughput under sterile conditions14. Lasers can be of varying wavelengths but are most commonly UV-B (280nm-315nm) or UV-C (100nm-280nm)14. The effects of thermal damage can also be investigated using IR-lasers. Localized heating of cell cultures simulates thermal-damage response leaving denatured ECM and cellular debris behind, creating a unique environment to which cells migrate10. As already mentioned for thermo-mechanical stamping, a downside of using heat is the reduced reproducibility due to heat spreading. A commercial research laser system for this purpose is the Stiletto® by Hamilton Thorne.
5. Electrical wounding
Electrical wounding is a technique that is based on the electroporation of cells by applying a local current via gold-film electrodes embedded in the culture vessel. By sending a high current through the electrode, cells on the surface are electroporated leading to cell death15. These electrodes can also measure the impedance in the electron flow caused by the cells in the culture vessel. The impedance is used as a measure for cell migration; the more cells migrate into the wound, the higher the impedance.
The advantages of this method include the high reproducibility of results because of the use of impedance measurements instead of optical measurements. This automated, real-time measurement excludes the possibility of errors due to human intervention16. However, the impedance measurements are easily influenced by changes in temperature, pH, or medium change15. Next to electroporating the cells, the electrodes could also cause local heat development that affects cell viability in the surrounding areas.
Currently, two commercial systems are available, the Electric Cell-substrate Impedance Sensing system (ECIS™) by Applied Biophysics and the xCELLigence by ACEA Biosciences. Both systems require the use of special (expensive) gold-coated well plates to be able to electrically wound the cells.
6. Chemical wounding
Chemical wounds can be created by chemical damage or removal of part of the cell monolayer. As cell dissociation reagents (e.g. trypsin) are essential in any research involving cell culture, chemical wounding can be performed in any cell culture lab. Localized wound areas can be created by adding a small droplet of the dissociation reagent to the cell culture.
To control the dimensions of the cell-free area, microfluidic devices are the norm for chemical wounding of cell cultures. A microfluidic device consists of two or more channels with inlets and outlets. Laminar flow prevents two different solutions (culture medium with and without trypsin) from mixing to detach only one part of the cell monolayer17. After detachment, the trypsin is removed, and cells can start to migrate into the void. This results in a fully integrated wound healing assay that could be precisely controlled18. The small volumes in microfluidics make these assays useful for studies with rare or costly compounds and cells17. One of the advantages of chemical wounding is the uniform matrix without substrate damage that is left in the cell-free area19. Other advantages of this system include the mechanical and chemical stimulation of cells by investigating the shear stress by fluid flow and by introducing chemical gradients in the channels19.
However, microfluidic devices can be quite demanding as daily medium changes are necessary because of the small volume of the medium inside the device. Because of this, vigilant control of the humidity in the incubator is also essential. Next to this, the successful use of microfluidic devices requires expertise. Many challenges can arise with microfluidic devices, including cell clumping, air bubble formation (prevents cells from growing), and leakage.
7. Vacuum-based wounding
A recently described method by De Ieso and Pei (2018) uses vacuum suction to remove an area of cells. In this way, a circular void is created using commonly available lab equipment (vacuum-pump and pipette tip)20. The benefit of this method is that the circular wounds are smaller than the field of view (FOV) of the microscope. This makes the relocation of the sample at each analysis timepoint more reproducible compared to that of linear voids. Another benefit is that cellular and ECM debris is removed when creating the void. This manner of damaging offers higher reproducibility than the manual scratch assay. However, since it is still a manual technique, it is less reproducible in wound size and geometry as opposed to automated cell removal techniques20.
Table 1. Methods of cell removal assays.
| Method | Technique | Advantage | Disadvantage | Commercially available products |
|---|---|---|---|---|
|
Scratch assay1,3 |
Mechanical |
Little to no requirements Easy to implement Cheap (manual) Fast |
Disrupts extracellular matrix (coating) Low reproducibility (manual) |
AutoScratch™ (BioTek) Cell Comb™ Scratch Assay (Merck) WoundMaker (IncuCyte®) HTSScratcher (Peira) Wounding Pin Tools (V&P Scientific, Inc.) |
|
Stamp wounding5,6 |
(Thermo)-mechanical |
Maintains substrate Cell debris removed with stamp High geometrical control |
Manual stamping affects reproducibility Thermal stamping impairs reproducibility No commercial products available |
- |
|
PDMS barrier11 |
Physical barrier |
Cells are wounded in a reproducible way Geometry of void is reproducible No substrate/matrix in void |
Difficult to automate No commercial products available |
- |
|
Laser-based wounding10,14,21 |
Radiation or thermal |
High reproducibility High throughput Custom shapes Sterile conditions |
Results of thermal ablation are difficult to reproduce High costs associated with acquisition of instruments |
Stiletto® (Hamilton Thorne) |
|
Electrical wounding15,22 |
Electroporation |
Submicroscopic resolution Real-time measurement Automated Quantitative and reproducible results |
Special equipment required Expensive culture vessels Difficult detachment of cell monolayers Local heat development that affects viability of cells |
ECIS (Applied Biophysics) xCELLigence (ACEA Biosciences) |
|
Chemical wounding17–19 |
Chemical |
Chemicals widely available No matrix damage Useful for rare/expensive compounds/cells (microfluidics) |
Droplet shape not reproducible Microfluidic devices required to control geometry Successful application of microfluidic devices requires expertise Daily medium change required |
- |
|
Vacuum-based wounding20 |
Mechanical |
Reproducible size Commonly available in cell lab Free from debris Higher reproducibility |
Variability in wound sizes and shapes |
- |
Related Products
There are currently no products tagged to this resource.